Q: .


- A. 10
- B. 8
- C. 13
- D. 15
Correct Answer:
Option D -
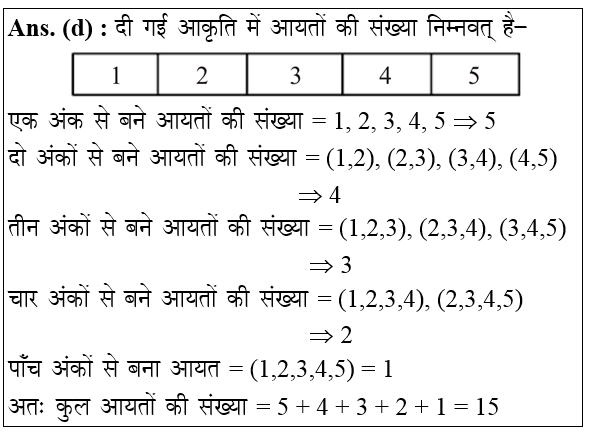

Explanations:



