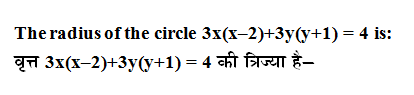Q: Which of the following is an example of colloidal solution of liquid in solid?
- A. Mud
- B. Milk
- C. Cheese
- D. Rubber
Correct Answer:
Option C - Colloidal solution
A colloidal solution is a heterogeneous solution which is made up of two phases; dispersed phase (as solute) and dispersion medium (as solvent).
The substance distributed as the colloidal particles is called the dispersed phase and the second phase in which colloidal particles are scattered is called dispersion medium.
When dispersed phase (liquid) is mixed with dispersion medium (solid), a new type of mixture formed is called as gel.
Example–Fruit jelly, cheese, butter etc.
C. Colloidal solution
A colloidal solution is a heterogeneous solution which is made up of two phases; dispersed phase (as solute) and dispersion medium (as solvent).
The substance distributed as the colloidal particles is called the dispersed phase and the second phase in which colloidal particles are scattered is called dispersion medium.
When dispersed phase (liquid) is mixed with dispersion medium (solid), a new type of mixture formed is called as gel.
Example–Fruit jelly, cheese, butter etc.
Explanations:
Colloidal solution A colloidal solution is a heterogeneous solution which is made up of two phases; dispersed phase (as solute) and dispersion medium (as solvent). The substance distributed as the colloidal particles is called the dispersed phase and the second phase in which colloidal particles are scattered is called dispersion medium. When dispersed phase (liquid) is mixed with dispersion medium (solid), a new type of mixture formed is called as gel. Example–Fruit jelly, cheese, butter etc.