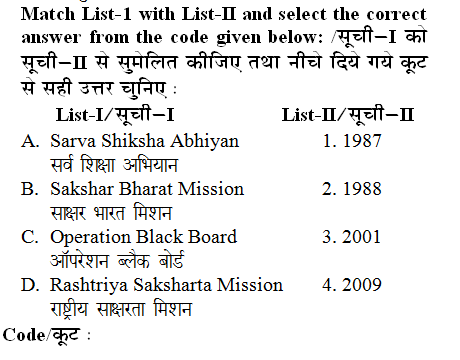
Q: Which of the following indicators has a transition point closest to the equivalence point for the titration of a weak acid by a strong base?
- A. Orange IV
- B. Thymol blue
- C. Methyl orange
- D. Bromcresol green
Correct Answer:
Option B - For the titration of a weak acid by a strong base, the equivalence point is typically in the basic pH range. Thymol has two transition range and the second range (around pH 8.0 to 9.6) in close to the pH at the equivalence point for this type of titration. Therefore thymol blue is the best choice among the given indicators.
B. For the titration of a weak acid by a strong base, the equivalence point is typically in the basic pH range. Thymol has two transition range and the second range (around pH 8.0 to 9.6) in close to the pH at the equivalence point for this type of titration. Therefore thymol blue is the best choice among the given indicators.
Explanations:
For the titration of a weak acid by a strong base, the equivalence point is typically in the basic pH range. Thymol has two transition range and the second range (around pH 8.0 to 9.6) in close to the pH at the equivalence point for this type of titration. Therefore thymol blue is the best choice among the given indicators.