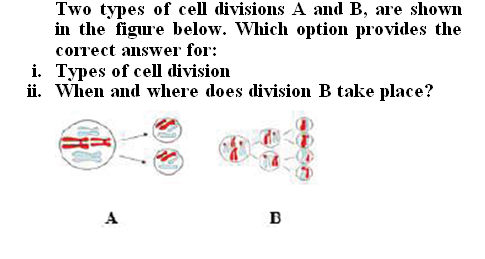
Q: Which law states that mass cannot be produced or destroyed in a chemical reaction?
- A. Law of Constant proportion
- B. Law of Inverse proportion
- C. Law of Energy conservation
- D. Law of Conservation of mass
Correct Answer:
Option D - The Law of Mass conservation was formulated by the French Chemist Antoine Lavoisier. According to this law, matter can neither be produced nor be destroyed in any chemical reaction, i.e. the mass of products in a chemical reaction is equal to the mass of the reactants.
D. The Law of Mass conservation was formulated by the French Chemist Antoine Lavoisier. According to this law, matter can neither be produced nor be destroyed in any chemical reaction, i.e. the mass of products in a chemical reaction is equal to the mass of the reactants.
Explanations:
The Law of Mass conservation was formulated by the French Chemist Antoine Lavoisier. According to this law, matter can neither be produced nor be destroyed in any chemical reaction, i.e. the mass of products in a chemical reaction is equal to the mass of the reactants.