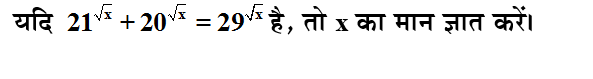Q: Which among the following is NOT true about a solution and the solute concerned?
- A. The particles of the solute settle down at the bottom when the solution is kept undisturbed.
- B. The particles of the solute do not scatter a beam of light.
- C. The particles of the solute cannot be seen with unaided eyes.
- D. A solution is a homogeneous mixture.
Correct Answer:
Option A - A composite mixture of two or more substance is called solution There are two major components of a solution - solute and solvent, the components of a solution that dissolves is called solute. Whereas mixture in which solute is dissolved is called solvent.
Properties of solution–
* It is a homogenous mixture.
* Its particle is smaller than 1 nm.
* Its particle cannot seen with naked eyes.
* They do not show tyndall effect.
A. A composite mixture of two or more substance is called solution There are two major components of a solution - solute and solvent, the components of a solution that dissolves is called solute. Whereas mixture in which solute is dissolved is called solvent.
Properties of solution–
* It is a homogenous mixture.
* Its particle is smaller than 1 nm.
* Its particle cannot seen with naked eyes.
* They do not show tyndall effect.
Explanations:
A composite mixture of two or more substance is called solution There are two major components of a solution - solute and solvent, the components of a solution that dissolves is called solute. Whereas mixture in which solute is dissolved is called solvent. Properties of solution– * It is a homogenous mixture. * Its particle is smaller than 1 nm. * Its particle cannot seen with naked eyes. * They do not show tyndall effect.