Q: The conjugate acid of HASO₄²- is-
-
A.
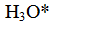
- B. ASO₄⁺
- C. H₃ASO ₄
-
D.
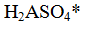
Correct Answer:
Option B - The conjugate acid of a species is formed when it gains a proton (H⁺). In this case HASO₄²⁻ gains a proton to become H2ASO₄⁻ making it the conjugate acid.
B. The conjugate acid of a species is formed when it gains a proton (H⁺). In this case HASO₄²⁻ gains a proton to become H2ASO₄⁻ making it the conjugate acid.
Explanations:
The conjugate acid of a species is formed when it gains a proton (H⁺). In this case HASO₄²⁻ gains a proton to become H2ASO₄⁻ making it the conjugate acid.
