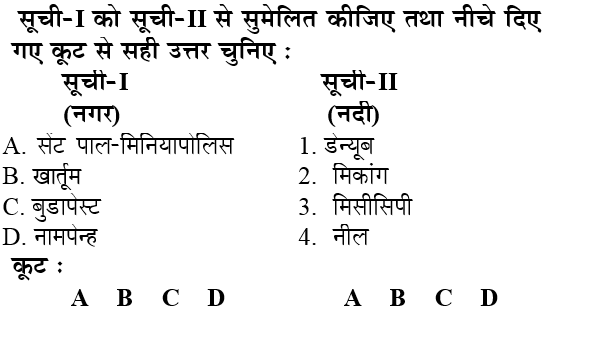
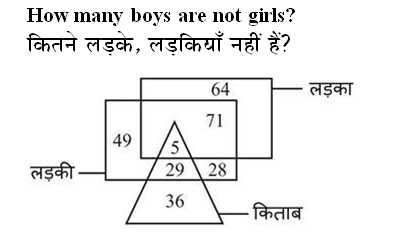
Q: कैलेंडर वर्ष के किस दिन को दुर्लभ रोग दिवस के रूप में स्वीकार किया जाता है?
- A. 18 फरवरी
- B. 28 मार्च
- C. 28 फरवरी
- D. 08 फरवरी
Correct Answer:
Option C - दुर्लभ बीमारियों के बारे में जागरूकता बढ़ाने के उद्देश्य से प्रतिवर्ष फरवरी माह के अंतिम दिवस 28 फरवरी को ‘रेयर डिजीज डे’ (Rare Disease Day) मनाया जाता है।
C. दुर्लभ बीमारियों के बारे में जागरूकता बढ़ाने के उद्देश्य से प्रतिवर्ष फरवरी माह के अंतिम दिवस 28 फरवरी को ‘रेयर डिजीज डे’ (Rare Disease Day) मनाया जाता है।
Explanations:
दुर्लभ बीमारियों के बारे में जागरूकता बढ़ाने के उद्देश्य से प्रतिवर्ष फरवरी माह के अंतिम दिवस 28 फरवरी को ‘रेयर डिजीज डे’ (Rare Disease Day) मनाया जाता है।