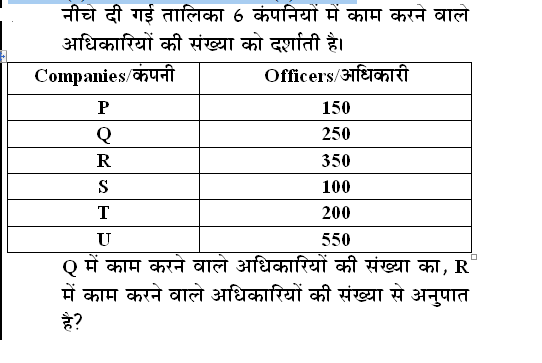
Q: The surface of a liquid acts like a stretched elastic membrane under tension. This is mainly due to ____________.
- A. viscosity
- B. velocity of flow
- C. surface tension
- D. capillarity
Correct Answer:
Option C - Surface tension and capillary effect:
• It occurs at the liquid- gas interface or at the interface of two immiscible liquid while a thin film is apparently formed due to attraction of liquid in the surface which is similar to tension in stretched membrane known as surface tension measured as (force/Length) unit (N/m).
• Surface tension is caused by force of cohesion between liquid molecules.
C. Surface tension and capillary effect:
• It occurs at the liquid- gas interface or at the interface of two immiscible liquid while a thin film is apparently formed due to attraction of liquid in the surface which is similar to tension in stretched membrane known as surface tension measured as (force/Length) unit (N/m).
• Surface tension is caused by force of cohesion between liquid molecules.
Explanations:
Surface tension and capillary effect: • It occurs at the liquid- gas interface or at the interface of two immiscible liquid while a thin film is apparently formed due to attraction of liquid in the surface which is similar to tension in stretched membrane known as surface tension measured as (force/Length) unit (N/m). • Surface tension is caused by force of cohesion between liquid molecules.