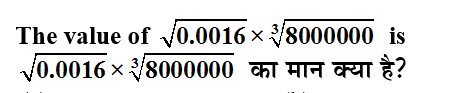Q: Temporary hardness in water is caused by
- A. Carbonates and bicarbonates of calcium and magnesium
- B. Bicarbonates of sodium and potassium
- C. Carbonates of calcium and potassium
- D. Dissolved carbon dioxide
Correct Answer:
Option A - Temporary hardness (Carbonate hardness)– It is caused due to the presence of carbonate and sulphate of calcium and magnesium. It is removed by boiling.
Permanent hardness (non carbonate hardness)– It is caused due to the presence of chlorides and nitrates of calcium and magnesium. It is removed by zeolite method.
A. Temporary hardness (Carbonate hardness)– It is caused due to the presence of carbonate and sulphate of calcium and magnesium. It is removed by boiling.
Permanent hardness (non carbonate hardness)– It is caused due to the presence of chlorides and nitrates of calcium and magnesium. It is removed by zeolite method.
Explanations:
Temporary hardness (Carbonate hardness)– It is caused due to the presence of carbonate and sulphate of calcium and magnesium. It is removed by boiling. Permanent hardness (non carbonate hardness)– It is caused due to the presence of chlorides and nitrates of calcium and magnesium. It is removed by zeolite method.