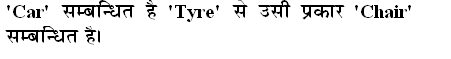Q: A reversible reaction in which a charged ion in solution is exchanged for a similarly charged ion electro-statically attached to an immobile solid particle is termed as:
- A. ion exchange
- B. carbonation
- C. efflorescence
- D. nitrification
Correct Answer:
Option A - Ion exchange is a chemical reaction in which free mobile ions of a solid, the ion exchanges, are exchange for different ions of similar charge in solution.
• The exchanger must have on open network structure. Either organic or inorganic. Which carries the ions and which allows ions to pass through it.
• Ion exchange process commonly used in industries and individual residence but it is not suitable for water with iron and Mn.
A. Ion exchange is a chemical reaction in which free mobile ions of a solid, the ion exchanges, are exchange for different ions of similar charge in solution.
• The exchanger must have on open network structure. Either organic or inorganic. Which carries the ions and which allows ions to pass through it.
• Ion exchange process commonly used in industries and individual residence but it is not suitable for water with iron and Mn.
Explanations:
Ion exchange is a chemical reaction in which free mobile ions of a solid, the ion exchanges, are exchange for different ions of similar charge in solution. • The exchanger must have on open network structure. Either organic or inorganic. Which carries the ions and which allows ions to pass through it. • Ion exchange process commonly used in industries and individual residence but it is not suitable for water with iron and Mn.